
If you've ever savoured the tang of yogurt, the richness of cream cheese, the sharp bite of pickles, or the complex flavours of miso, tempeh, or kimchi, then you've tasted the magic of fermentation.
This isn’t just a culinary technique. It’s an ancient ritual, passed down through generations, harnessing the invisible power of microbes to transform and preserve.
What begins as humble ingredients are reborn, enriched, and with a good shelf life. Fermentation has been the guardian of our food, keeping it alive and thriving through the ages.
Today, food technology has improved dramatically, aided by "precision fermentation”.
Multi-billion food production trend
It’s the newest trend among food tech investors, driving a multi-billion growth in an industry known as “alternative protein production”.
Precision fermentation is being used to produce a variety of alternative proteins, including plant-based meat and dairy alternatives.
What are the benefits?
Let’s start with insulin, which is medicine, not food.
About 50 years ago, in the 1970s, insulin was made from animals. It was extracted from animal pancreases – a process needing about 50,000 animals to harvest a staggering 10,000 pounds of animal material for just one pound of insulin.

Some techniques required even more glands per unit of insulin (IU). By 1981 production of one pound of insulin required 8,000 pounds of glands from 23,500 animals — but this was only enough insulin to treat 750 patients with diabetes for one year.
It’s not cheap.
Disruption of food and agriculture
In 1982, human insulin was made using modified yeast, via recombinant DNA technology. The process was developed by Genentech and licensed as well as marketed by Eli Lilly.
This was a game-changer, replacing animal-based insulin with a human-identical version.
Stanford University researcher Tony Seba coined the term “precision fermentation” in his 2019 report with Catherine Tubb.
Now, using the same human insulin production process to make specific proteins using microorganisms genetically modified to create the desired product — whether it’s milk protein, meat, or leather — has emerged.

And investors are making big bets on the technology, according to Fortune Business Insights.
The global precision fermentation market size, valued at $2.14 billion in 2023, is projected to hit $57.01 billion by 2032, an annual growth rate of 44.3 per cent. Europe dominated the precision fermentation market with a market share of 49.53 per cent in 2023.
$ 3.03 b
global precision fermentation market value in 2024 (Source: Fortune Business Insights)Ending world hunger?
Could precision fermentation usher in the next revolution,a nd could it help end world hunger?
Despite the advanced food tech available today, an estimated 2 billion people across the world still face “hidden hunger” — a form of malnutrition characterised by micronutrient deficiencies.
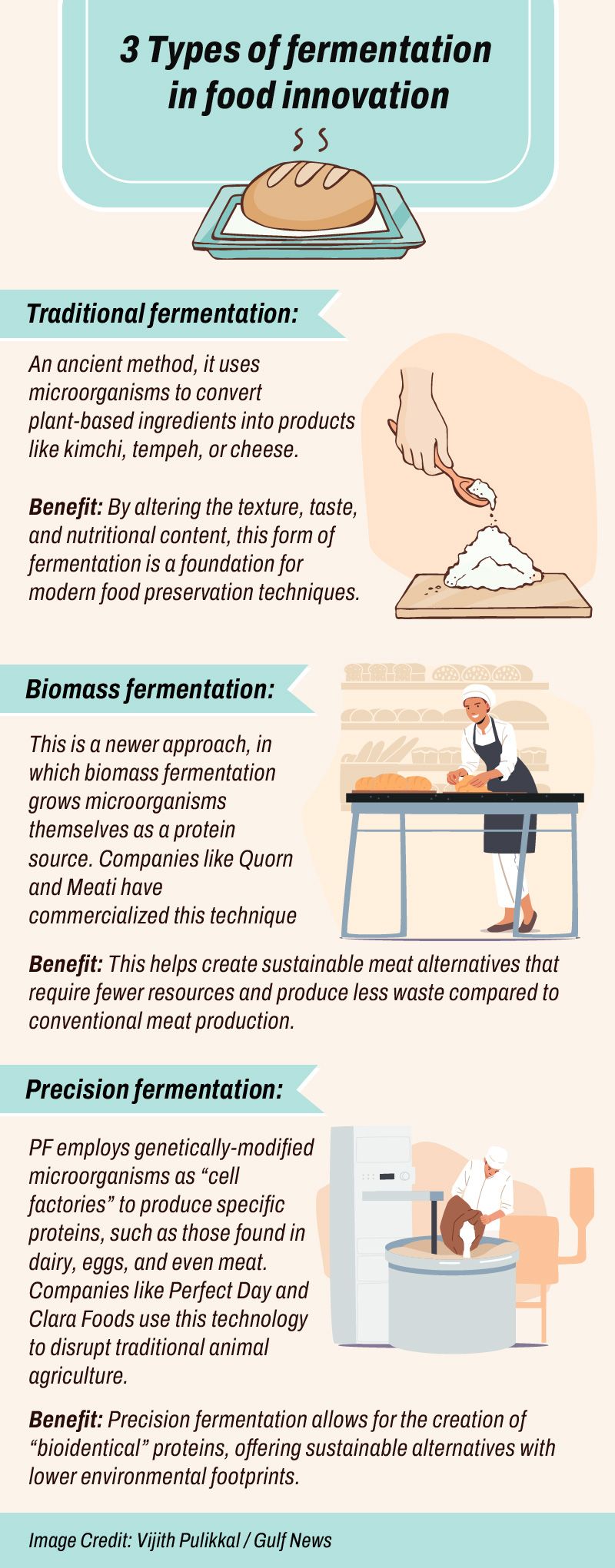
Precision fermentation
As food systems face increasing pressures from climate change and rising populations, PF is emerging as a key tool for transforming the way we produce and consume food.
PF is now leading innovations across various sectors, from alternative proteins to sustainable biofuels.
2 b
estimated number of people affected by the so-called 'hidden hunger' around the world.This same precision fermentation technology is now being used to disrupt the dairy industry.
Unlike overt hunger, hidden hunger is often invisible, as individuals may consume enough calories but lack essential vitamins and minerals.
Milk proteins like casein and whey are being produced without cows, offering a direct replacement for animal-derived dairy ingredients.
These precision fermentation proteins are chemically identical to traditional milk proteins, providing the same taste and functionality in products such as cheese, yoghurt, and protein bars.
Benefits
The economic benefits are striking.
The cost of producing precision fermentation proteins has dropped dramatically, from $1 million per kilogram to just $100 per kg in recent years.
According to some projections, the cost could drop to as low as $1 per kilogram by 2030, outcompeting traditional dairy on price and scalability.
Seba predicts that this disruption will start as early as 2025, with a collapse of the dairy industry driven purely by economic factors .
Its purpose is to provide a safe and inexpensive means for the mass production of recombinant pharmaceutical proteins (such as antibodies, subunit vaccines, and enzymes) which may may offer a cheaper way of making biotech drugs and vaccines than traditional factory systems.
(Source: National Institutes of Health)
Impact
Fermentation farms, driven by precision biology, can be located in or close to towns and cities.
The impact of precision fermentation goes far beyond food.
As a more resource-efficient process, it could free up to 2.7 billion hectares of land currently used for livestock agriculture, according to Seba, author of Rethinking Food and Agriculture 2020-2030: The Second Domestication of Plants and Animals.
This land, equivalent to the size of the US, China, and Australia combined, could be repurposed for reforestation and carbon sequestration.
Precision fermentation uses just 1 per cent of the land and water required by conventional animal farming, significantly reducing greenhouse gas emissions and minimising deforestation .
Countries that currently rely on large-scale livestock farming may need to adapt to this new reality, while others with expertise in biotechnology could emerge as leaders in the next generation of food production.
This shift in food production will have wide-reaching geopolitical consequences, as the global food system is restructured.
These databases then become “molecular cookbooks” that food engineers anywhere in the world can use to design products in the same way that software developers design apps. Novel food systems will be decentralised, stable and more resilient than current industrial agriculture.
The food-as-software model allows for constant iteration so that products improve rapidly, with each version superior and cheaper than the last.
Downsides of precision fermentation
There a a number of downsides to PF downside. The biggest one:
Cost
The specialised bioreactors required for growing lab meat are costly to design, build, and operate. Lab-grown cells also require a precise and expensive nutrient mixture, making production more costly than traditional livestock farming.
At the moment, even with advancements in production efficiency, lab-grown meat is likely to be more expensive than traditional meat for the foreseeable future. Scaling up lab meat production to meet global demand would also require huge investments and technological advancements.
Growing stem cells in bioreactors also produce waste (lactic acid and ammonia), so the cell media has to be periodically circulated and filtered or replaced, and the waste must be disposed of.
Regulation
Precision fermentation products must undergo rigorous safety testing and approval from food safety authorities before entering the market.
Moreover, regulatory frameworks differ across countries, creating challenges for global commercialisation. These processes can be time-consuming and may slow down the adoption of these innovations.
Consumer acceptance
Public perception of lab-grown or microbe-produced food can be a significant barrier. Many consumers may be hesitant or uncomfortable with the idea of eating food produced by genetically engineered microorganisms. Addressing concerns about GMOs, naturalness, and food safety will be crucial in gaining consumer trust and acceptance. Additionally, labelling and transparency will play a key role in educating the public.
Energy and resource demand
While PF reduces the need for land and water compared to traditional agriculture, it can still be energy-intensive. The fermentation process requires precise control over environmental conditions (temperature, pH, oxygen levels), which are energy hogs. More efficient and renewable energy sources will be essential for maximising the environmental benefits of this technology.
Corporate control
The PF sector is driven by biotechnology and innovation, which often leads to patenting microorganisms, processes, and products. This can lead to issues around corporate control and monopolisation. A few large companies could dominate the market and increasing prices. There is also concern about equity in access to these technologies, particularly in developing nations.
Competition with traditional agriculture
Precision fermentation could disrupt traditional agriculture, leading to potential job losses in farming communities, particularly those involved in livestock and dairy production. This shift may create socioeconomic challenges, especially in regions heavily dependent on conventional agriculture for livelihoods. Transitioning these communities to new economic opportunities will be critical to mitigate the negative impacts.
The path forward
As precision fermentation continues to scale, it offers a glimpse into a future where food is produced in ways and at a scale never been done before.
Its potential to disrupt entire industries — from agriculture to medicine — marks one of the most significant advancements since the first domestication of plants and animals over 10,000 years ago.
Would PF be more sustainable, ethical, and efficient than traditional methods?
Would it really meet the growing demand for food and other products, and solve the so-called hidden hunger affecting billions?
Does it really address food security and sustainability challenges?
Certain PF benefits are already clear, especially for medicine and agriculture. Given the huge investments into PF research and development, the industry is poised for significant growth.
By continuing to develop and scale this technology, we may be able to unlock new possibilities for a healthier planet and a more secure food system. The key challenge today: navigating the economic and societal changes that come with this revolution.











