
Highlights
- Experts say the therapeutic applications of base editing are just dawning.
- New T-cell therapies are helping revolutionise the treatment options for cancer patients.
In December, a teenage girl’s “incurable cancer” was cleared from her body using gene-editing technology. Alyssa, a 13-year-old from Leicester, received genetically-engineered immune cells known as “CAR T-cells".
She is the first of 10 people to be given the treatment as part of a UK clinical trial (drug study conducted on humans to measure safety and efficacy).
It’s a fascinating new field in medicine. Unlike surgery, chemotherapy and radiation therapy, the relatively new gene editing technique helps redirect the immune system to fight cancer. CAR T-cells therapy has increasingly become a standard treatment for patients with aggressive lymphomas, thus becoming a part of modern medicine.
What we know so far:
What are CAR T-cells?
They are T-cells, or T-lymphocytes, which are immune cells in humans genetically engineered to express a chimeric antigen receptor (CAR) — an artificial receptor that helps kill cancer cells.
In general, genetically altering (or “editing”) T-cells to express a CAR enables the immune system to target and destroy specific cancer cells' surface proteins.
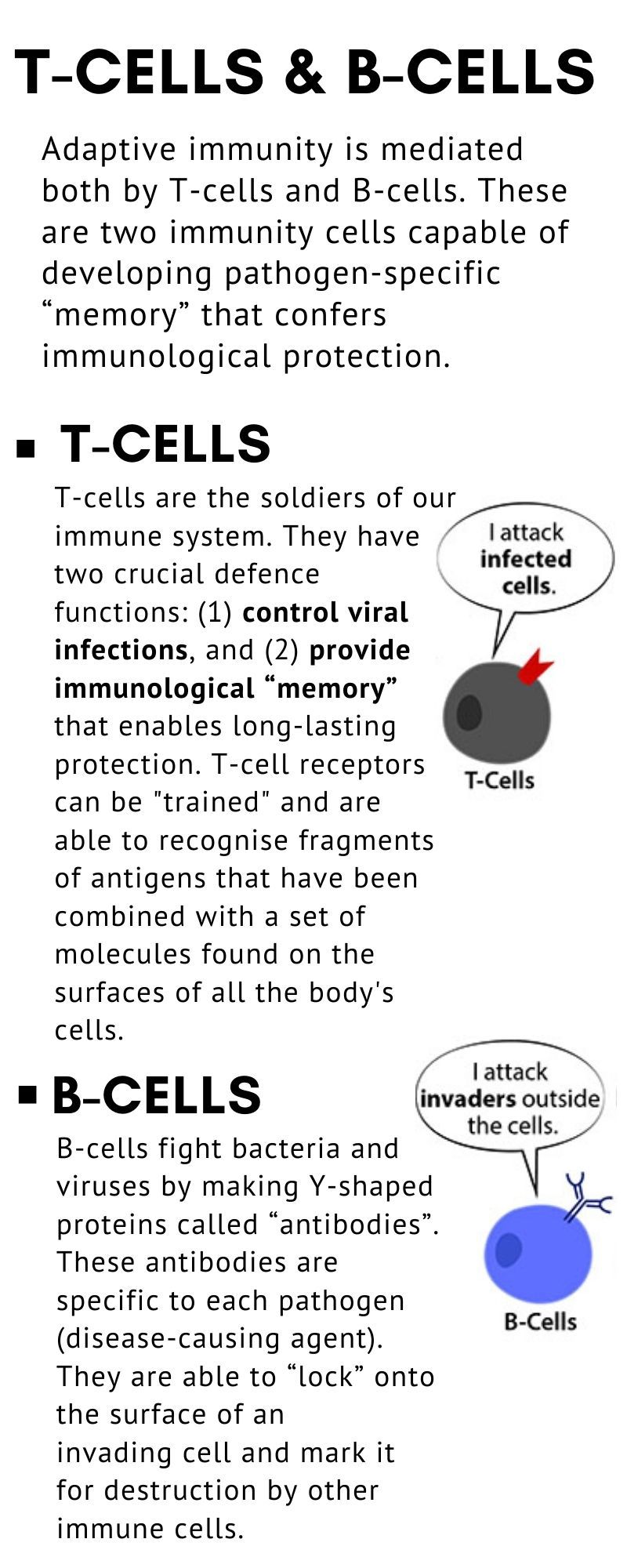
• There are two main types of lymphocytes: T cells and B cells.
• B cells produce antibody molecules that can attach to — and destroy — invading viruses or bacteria.
• T cells, produced by the thymus gland, are direct fighters or “killers” of foreign invaders.
• They also produce cytokines, which are biological substances that help activate other parts of the immune system. Lymphocytes include the main type of cell found in lymph, which prompted the name “lymphocyte”.
What is immunotherapy?
Immunotherapy is a type of treatment being actively investigated to treat different cancer types. In a nutshell, it uses substances made by the body, or prepared in a lab, to boost the immune system and help the body find — and destroy — cancer cells.
Immunotherapy can be used alone, or in combination with others including chemotherapy, radiotherapy and other cancer treatments.
How does CAR T-Cell therapy work?
CAR T-Cell therapy is an immunotherapy in which T-cells are “reprogrammed” to seek out and destroy cancer cells. As a result, these reprogrammed T-Cells (or T-lymphocytes) operate like “living medicines”.
They move throughout the body, continually stimulating the patient’s immune system to fight the disease.
Why did Alyssa sign up for CAR T-cell therapy?
In 2021, Alyssa was diagnosed with T-cell acute lymphoblastic leukaemia (T-ALL) and received all conventional treatments for her cancer — including chemotherapy and a bone marrow transplant. Her disease returned. That left her and the doctors with no further treatment options.
Surgery, chemotherapy, radiation therapy, bone marrow transplant have been the cornerstones of cancer treatment for decades. It's only within the last six years that immunotherapy treatment categories, including T-cell therapy, have expanded the treatment landscape for cancer patients.
Typically, a patient’s own T-cells are typically used in CAR-T therapy. To enable the T-cells to locate and eradicate cancer cells, the T-cells are modified or “edited.”
In Alyssa’s case, however, the T-cells were the primary driver of her blood cancer condition, in which cells developed to identify and destroy malignant T-cells kill one another as they are being “manufactured” before being used as a treatment. That’s when her parents and Alyssa herself decided to sign up for the clinical trial of the new CAR T-cell therapy.

What is new with CAR T-cell cancer treatment?
Researchers have been hard at work to develop a new type of CAR T-cell therapy that specifically attacks cancerous T-cells. They use a method called “base editing” to change single letters of the DNA code in the T-cells.
Essentially, it involves editing CAR T-cells before they can be given to the patient. The procedure allows the T-cells to be “programmed” so they do specific tasks, such as destroy cancerous T-cells in the body.
Following successful treatment, the patient then receives a bone marrow transplant to restore their immune system.

What happened in Alyssa’s case?
In May 2022, Alyssa became the first patient to join a clinical trial at Great Ormond Street Hospital (GOSH).
T-cells from a healthy donor that had been modified with the new technique were used to treat her. Four weeks later — at Day 28 — she was in remission. Then, in order to rebuild her immune system, she underwent a second bone marrow transplant. Currently, she is doing well at home, recovering with her family, six months after the bone marrow transplant, British media reported.
Who administered the therapy?
The base-editing therapy was developed by a team led by the National Institute for Health and Care Research (NIHR), a British government research arm.
Senior scientist Waseem Qasim, a member of the NIHR GOSH Biomedical Research Centre, who received funding for related studies, said: “This is a great demonstration of how, with expert teams and infrastructure, we can link cutting-edge technologies in the lab with real results in the hospital for patients.”
“It’s our most sophisticated cell engineering so far and paves the way for other new treatments and ultimately better futures for sick children.”
Dr Steven Rosenberg, chief of the Surgery Branch in NCI’s Centre for Cancer Research (CCR), an immunotherapy and CAR T-cell therapy pioneer, said CAR T-cell therapies have entered the mainstream of cancer treatment.
Some patient advocates have called for CAT T-cell therapy to be the main line of defence, especially against childhoood cancer, instea of the last.
A patient’s T cells, a subset of immune cells, are typically used in CAR T-cell therapy to combat cancer cells. To enable the T-cells to locate and eradicate cancer cells, the T-cells are modified, or “edited,” in the laboratory. However, the procedure could not function normally because renegade T-cells were the root cause of Alyssa’s malignancy. Prior to being administered as a treatment, T-cells created to recognise and eliminate malignant T-cells also kill each other during the “manufacturing” process.
How does CAR T-cell therapy help fight cancer?
At the moment, it has been used in humans with certain types of cancers, and only on an experimental basis. It has been trialled on people with sickle cell disease and beta-thalassemia, who carry defects in the gene for haemoglobin, the oxygen-carrying protein in the blood.
As an experimental treatment, the treatment given to Alyssa has not been approved for wider use, yet.
Who are the leading entities conducing CAR T-Cell therapy trials?
Clinical trials have been conducted by two biotech companies: Vertex Pharmaceuticals and CRISPR Therapeutics. In those trials, blood stem cells from the patient are removed, CRISPR gene-editing technique is used to turn on a healthy gene for foetal haemoglobin, which cells shut off after birth, and the changed cells are then reinfused.
Dr Steven Rosenberg, chief of the Surgery Branch in NCI’s Centre for Cancer Research (CCR), an immunotherapy and CAR T-cell therapy pioneer, said CAR T-cell therapies have entered the mainstream of cancer treatment.
Is T-cell therapy available in the UAE?
In September 2021, the Abu Dhabi Stem Cells Centre (ADSCC), a leading UAE medical research institute, announced they are studying the safety and effectiveness of chimeric antigen receptor (CAR) T-Cell therapy to combat cancers like myeloma, lymphoma and certain forms of leukaemia.
• Lymphoma
• Some types of leukaemia
• Multiple myeloma
How much is CAR T-cell therapy?
According to Prime Therapeutics, a US-based pharmacy benefit manager, CAR-T cell treatment costs may range from $700,000 to $1 million. The US National Cancer Institute reported that the most recently-approved CAR T-cell therapy costs more than $450,000.

T-Cell receptor (TCR) Therapy
A new immuno-therapy, known as adoptive T-cell receptor (TCR) therapy, is also under investigation, this time against solid tumours.
A clinical trial whose results were published January 9, 2023 in the journal Nature Medicine shows an adoptive TCR therapy known as afami-cel (Afamitresgene autoleucel, formerly ADP-A2M4), achieved “clinically significant results” for patients with multiple solid tumour types.
The trial, a Phase I study, was led by researchers at The University of Texas MD Anderson Cancer Center. It included 38 patients — 58% male; 92% were white and the rest were Asian — treated with afami-cel, with an average of three prior lines of therapy.
The study included 16 patients with synovial sarcoma, nine with ovarian cancer, three with head and neck cancer, two each with esophageal cancer, non-small cell lung cancer, urothelial cancer and myxoid/round cell liposarcoma, and one each with gastric cancer and melanoma.
Results show that in the subgroup of patients with synovial sarcoma, afami-cel TCR achieved “objective response rate” of 44 per cent compared to the overall response rate of 24 per cent across all cancer types.
These high response rates are significant as patients with synovial sarcoma really have very few options after high-dose chemotherapy with ifosfamide, according to Dr. David S. Hong, professor of Investigational Cancer Therapeutics and principal investigator in the study.
Dr. Hong said the overall toxicity from afami-cel was “manageable”, adding that researchers also saw evidence of early activity in other cancer types.
“These results suggest this is an approach with the potential to work in solid tumours — where there are currently no approved cellular therapies,” said Dr. Hong.










