Camels may not be the first creatures that come to mind when you think about medical breakthroughs.
But these fascinating humped animals, characterised by their long, slender necks and legs with padded feet, hold the key to a new class of medicine thanks to the discovery of nanobodies – tiny antibodies found in their blood.
The serendipitous discovery of nanobodies, their unique properties, shows the potential to append medical science.
Camel nanobodies: A medical revolution
It’s a fascinating story. “Nanobodies” are called such as they are tiny antibodies derived from camels, llamas, and alpacas – with potential to revolutionise medicine.
Today, nanobodies derived from camel blood are at the forefront of a medical breakthrough, with potential applications ranging from combating infectious diseases to diagnosing Alzheimer's disease.
Known for their ability to penetrate small areas and bind precisely to pathogens, nanobodies are now being explored for various therapeutic uses.
Accidental discovery
The journey began in the 1990s at the Free University of Brussels. Due to student reluctance to donate blood, researchers used dromedary blood in an experiment.
This unexpected choice led to the discovery of antibodies with a much lower molecular weight than previously seen.
Professor Raymond Hamers and his team, including Serge Muyldermans, pursued further investigations, confirming the presence of these “mini-antibodies” in all camelids.
Unique properties: Tiny yet powerful antibodies
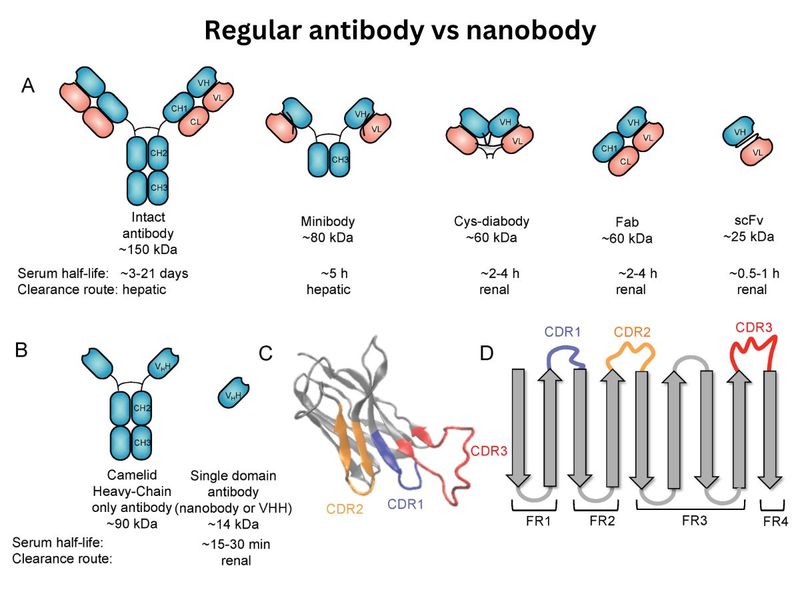
What makes these nanobodies special? Their small size allows them to penetrate areas inaccessible to larger antibodies.
Additionally, they are remarkably robust and retain their binding capacity even when fragmented. This makes them highly desirable for therapeutic applications.

Discovery to development
Following the initial discovery, researchers needed to confirm the functionality of these nanobodies.
They successfully immunised a camel and observed the production of mini-antibodies targeting the antigen.
Muyldermans’ crucial finding was the ability to isolate the highly effective binding tip (nanobody) from the rest of the antibody molecule. This tiny fragment remained ultra-resistant with intact binding capabilities.
Patenting and publication
Muyldermans, Hamers, and Cécile Casterman secured a patent for their discovery in 1993, paving the way for commercial development.
The groundbreaking research was then published in the prestigious journal Nature, attracting global attention from scientists and laboratories.
Unlocking the potential
Researchers like Alain Roussel in France began unraveling the structure of nanobodies, revealing three key loops that grasp the antigen with exceptional precision.
This atomic-level confirmation solidified the effectiveness of nanobodies as binding agents.
The spotlight on nanobodies exemplifies the power of serendipity – chance discovery – in scientific advancements.M uch like other accidental breakthroughs such as aspirin and X-rays, this unexpected finding stemmed from a combination of chance and scientific curiosity.
Medical research in camel nanobodies is an exciting field. For example, scientists are now using nanobodies for super-resolution microscopy. Nanobodies' tiny size allows them to reach areas inaccessible to regular antibodies, revealing cellular processes deep inside.
Researchers Léa Berland and Omar Abousaway (who both work for Department of Cancer Immunology and Virology, Dana-Farber Cancer Institute, Boston), published their work in the journal Biomolecules (MPDI) in April 2021 on the use of nanobodies for medical imaging.
To get a more accurate view of cancer tumours, researchers said the use of full-sized antibodies present certain limitations as they are unable to efficiently penetrate the entirety of the tumour, leading to an incomplete visualisation of the lesion.
On the other hand, they cited single-domain antibody fragments, referred to as VHHs, or nanobodies present as "attractive alternatives".
Tumour blaster
Research published in the journal Nature Communications in September 2023 shows how camel nanobody-based B7-H3 chimeric antigen receptor T (CAR-T) demonstrated “high efficacy” against large solid tumours.
Scientific interest has snowballed such that more than 20 clinical trials including nanobody CAR-T therapy targeting B7-H3 are in progress.
The technology holds immense promise. One use case is understanding diseases at their root cause; another is developing new treatments.
Therapeutic applications
While Muyldermans focused on fundamental research, others explored the therapeutic potential of nanobodies.
Antonella Fioravanti's research at the Free University of Brussels investigated the use of nanobodies to neutralize anthrax toxins. Initial studies showed promising results in blocking the receptor and preventing anthrax absorption.
However, further research is needed to translate these findings into a viable treatment.

Beyond treatment: Diagnostics and imaging
Nanobodies offer applications beyond therapeutic interventions. Their ability to bind specifically to antigens makes them valuable tools for disease detection.
Additionally, scientists are using nanobodies, tagged to make them fluorescent – to image cellular functions at a higher resolution than previously possible.
Researchers at the University of Göttingen are pioneering the use of nanobodies for super-resolution microscopy, allowing for detailed visualisation of cellular processes.
Future of nanobodies
The potential applications of nanobodies seem limitless, say scientists. They hold promise for fighting cancer, diagnosing Alzheimer's disease, and various other medical advancements.
This discovery, sparked by a fortunate accident, continues to revolutionise the field of medicine.
• CAR-T therapy: Chimeric Antigen Receptor T-cell therapy. It involves engineering a patient's own T cells to recognise and attack cancer cells.
• B7-H3 receptor: A protein found on the surface of many tumour cells and some normal tissues. High levels of B7-H3 are associated with poor prognosis in cancer patients.
• Targeting B7-H3: CAR-T cells are designed to recognize and bind to the B7-H3 receptor on cancer cells. This triggers the T cells to kill the cancer cells.
Potential benefits:
• Specificity: B7-H3 is overexpressed on some tumors, potentially allowing CAR-T cells to target cancer cells while minimizing harm to healthy tissues.
• Potency: Preclinical studies show CAR-T cells targeting B7-H3 can be highly effective against various solid tumours.