As the world races to find a cure for the deadly novel coronavirus, a Kolkata woman is playing a crucial part in the team at the University of Oxford that is trying to develop a COVID-19 vaccine.
Chandra Datta is a 34-year-old who lives in Oxford, and she works as a quality assurance manager at the university that manufactures the vector antiviral vaccine, ‘ChAdOx1 nCoV-19’, which was tested in humans last recently.
According to Indian news reports, if the vaccine passes the trials, it could be made available to the public by September or October this year, Datta said, adding: “It all depends on the data from the trial.”
Datta, originally from Kolkata, attended Gokhale Memorial Girls’ School and completed her bachelor’s degree in engineering and biotechnology at the Kolkata Institute for Technological Heritage.
She moved to the UK in 2009 to take the MSc-Bioscience (Biotechnology) course at the University of Leeds.
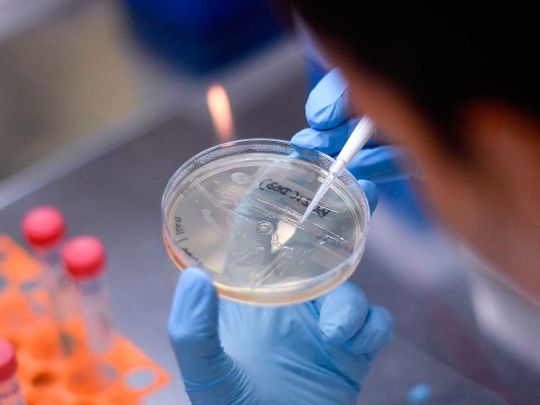
Datta currently holds a position at the clinical bioproduction factory at the University of Oxford, which produces viral vector vaccines for early phase clinical trials worldwide.
Catherine Green, @CathGreenLab, who describes herself as “Associate Professor in Chromosome Dynamics at the Wellcome Centre for Human Genetics at University of Oxford and Head of the Clinical Bio-manufacturing Facility (CBF)” tweeted about the team's efforts: “We made it! The first batch of ChAdOx1 nCoV-19 vaccine that is being used in the clinical trials here in Oxford was manufactured by my marvellous team at the CBF. We take no credit for the conception or the design, but we will take credit for having gone from DNA construct to […] GMP certified product into trial in 65 days.”
In consecutive tweets she thanked her team members, including Datta.
Datta oversees the quality assurance aspect of vaccine manufacturing, ensuring that procedures and methods are compliant to standards.
Times of India quoted Datta as saying: “After checking all the documents, the quality professional certifies the lot that he is happy that the vaccine is subject to a clinical trial. It happened on Wednesday with the COVID-19 vaccine.”
“It was an incredible experience,” she added.
“Last month everyone was under great pressure, but we did it very quickly. There has been a huge team effort,” she told Times of India.
“From what I have heard, we plan to start mass production at the Serum Institute in Pune before the trial ends. Once the trial is successful, it can be marketed,” she added.
“Vaccine development normally takes three to four years to develop a vaccine and we are trying to do it in a few months. So far we have made approximately 600 of the vaccines. We are in the process of manufacturing more. I think we can make 1,000 and then it will get mass produced. They are studying new manufacturing facilities in the UK that have not yet been finalised, “she said.
The Oxford team plans to vaccinate 800 volunteers in the UK over the next month. If the trial is successful, they will ask the government of Kenya for permission to carry out an assessment in Kenya, Times of India reported
“I think the UK will be the first to have it obviously since it is a British invention. The vaccine will need to be given to the world if enough manufactured vaccines are available, ”Datta was quoted as saying.
Indians took to social media to praise her for her contributions.
Indian Politician Derek O’Brien, @derekobrienmp, tweeted: “So proud of Chandra Dutta, 34-yr-old quality assurance manager from Kolkata, part of an Oxford University team developing a #COVID19 vaccine Kudos to 100s like her who are working round the clock to overcome this challenge.”
Tweep @IraniDarayas: “May Chandra Datta's Oxford University team creating the anti-COVID-19 vaccine see success soon for the good of the human race. History go with you!”








